Where Can I Buy Ammonia For Cleaning
Abstruse
There is scant data pertaining to airborne ammonia exposures from either spills or common household uses of ammonia-containing floor and tile cleaners or from spray-on drinking glass cleaners. We assessed instantaneous and event-specific time-weighted average (TWA) exposures to airborne ammonia during spills and use (per label directions) of a household floor and tile cleaner and two spray-on window cleaners. Airborne ammonia levels measured at animate zone height (BZH) above the spilled floor and tile cleaner production reached 500 p.p.m. inside five min, while levels for spilled window cleaner were below 8 p.p.m. TWA exposures were assessed while tile walls and floors were cleaned in 3 different bathrooms of a residence, and during use of a spray-on glass cleaner while washing several large windows in an function setting. NIOSH Method 6015 was utilized with concurrent field measurements every lx due south using a Drager PAC 3 monitor with an electrochemical prison cell detector. Peak ammonia levels ranged from xvi to 28 p.p.g. and short-term TWA concentrations ranged from ix.4 to xiii p.p.m. during mixing (0.ane% ammonia) and cleaning tiles in the iii bathrooms. Ammonia exposures while using spray-on window cleaner were over x-fold lower (TWA=0.65 p.p.one thousand.). Use of the floor and tile cleaner mixed at 0.2% ammonia led to peak airborne ammonia levels inside 3–five min at 36–ninety p.p.m., and use of full forcefulness cleaner (3% ammonia) led to tiptop ammonia levels of 125 to >200 p.p.1000. within 2–3 min. Spillage or intentional apply of the full forcefulness flooring and tile cleaner led to airborne ammonia concentrations that exceed occupational short-term exposure limits, while spillage or use of the spray-on window cleaner did not approach potentially hazardous airborne ammonia levels and likely represents a minimal inhalation health hazard. We conclude that routine household uses of ammonia are unlikely to produce significant exposures when using standard cleaning solutions (0.ane–0.2%), only spillage or use of full-bodied ammonia solutions (e.m., 3%) in poorly ventilated areas can atomic number 82 to potentially chancy airborne ammonia exposures.
Introduction
Ammonia is a widely used hazardous chemical with many potential applications in agronomics, industry, and commercial products, including various household cleaning products. The most probable source for ammonia exposure in the general population is from the use of household cleaners containing ammonia or ammonium salts (ATSDR, 2002). Ammonium hydroxide ("ammonia") is the primary active amanuensis for cleaning and disinfecting nonporous surfaces in various domestic, commercial, and "industrial forcefulness" cleaning products. Its widespread utilise and the popular knowledge of ammonia as a strong cleaning and disinfecting amanuensis leads to a strong potential for use of relatively concentrated forms by individuals who may not exist fully informed about the potentially serious inhalation health hazards associated with improper uses of this chemical. Nazaroff and Weschler (2004) reviewed the risk potential of a variety of cleaning products, including certain ammonia-containing cleaners.
As explained later in detail (in the "Review of Dose–Response Considerations" section), ammonia is a well-recognized corrosive amanuensis and sensory irritant with relatively practiced warning properties due to its pungent odor and the irritation effects of airborne exposure, which promote abstention of connected exposure. Although there are well-known serious health consequences from oral and dermal exposure to concentrated ammonia solutions (ATSDR, 2002), the focus of the present written report is on inhalation exposures and associated health risks. Acute inhalation exposures are of item importance considering ammonia dose–response relationships demonstrate a relatively steep dose–response that is highly dependent on "concentration times duration," that is, cursory, high exposures are typically most hazardous (NAS, 1987; NIOSH, 1997; AIHA, 2001; ATSDR, 2002). Moreover, many private-specific factors such as age, smoking history, and underlying chronic lung diseases can influence the severity and permanence of ammonia-induced lung damage or functional deficits (WHO, 1986; NAS, 1987; ATSDR, 2002).
The purpose of the current report was to examine in a quantitative fashion the nature and extent of ammonia exposure during household use and spills of selected ammonia-containing cleaning products. Two types of cleaning products were examined: (ane) a floor and tile cleaning concentrate solution stated to contain 5% ammonium hydroxide, and (2) a spray-on drinking glass cleaning solution stated to contain 1–three% ammonium hydroxide. Instantaneous and TWA airborne exposures to these products were simulated during normal use and adventitious spill scenarios. We likewise provide an overview of the dose–response considerations regarding ammonia inhalation exposures and a product-specific hazard assessment to identify our exposure assessment findings in perspective with the available human studies on health hazards of inhaled ammonia.
Methods
Products Assessed
The household ammonia-containing cleaners that were evaluated in the current study include a typical "store brand" ammonia concentrate (v% ammonia past volume per the product characterization) sold for general use equally a floor and tile cleaner, too as prepare-to-use formulas of a "name brand" drinking glass cleaner (1% and iii% ammonium hydroxide per the material safety information canvas) and a "store brand" glass cleaner (ammonia content non stated). There are many like proper noun brand and store brand products to these offered at grocery stores and other stores throughout the United states. The floor and tile cleaner product was purchased in a 64-ounce volume in a plastic container with a screw cap pinnacle. The window cleaner products were purchased in 32 ounce plastic bottles with a mechanical pump sprayer.
Product Ammonia Content Determination
Each of the iii products was evaluated for pH and total dissolved nitrogen content at a commercial laboratory (Galbraith Laboratories, Knoxville, TN, USA) certified for commercial products analysis by Good Manufacturing Practices (GMP) certification, as well every bit Good Laboratory Practices (GLP) and USEPA laboratory certifications. The pH was adamant past using an Accumet pH Meter, Model 25. Dissolved ammonia concentrations were assessed by determining the dissolved nitrogen content in each solution using the Total Kjeldahl Nitrogen method (Bradstrut, 1965). Duplicate samples were evaluated for two of the 3 products assayed. Since the solution pH was approximately 11 in each production, essentially all of the dissolved nitrogen was likely to be in the course of ammonia. Dissolved ammonia concentrations were calculated past multiplying the dissolved nitrogen results (mg/l) by 1.43 to correct for the molecular weight difference between ammonia and nitrogen (ten/7=1.43).
Spill Exposure Studies
A pilot study was conducted to examine exposures created by a simulated accidental spill wherein the newly opened product container was tipped over and allowed to spill out on a level surface (within a bathtub with the drain plugged). This procedure caused approximately one-half of the container to spill out, and the volume spilled for each container was assessed as the average of three trial spill simulations using the same containers filled with the same volume of water and measuring the amount remaining in the container. Drager colorimetric tubes (Drager-Rohrchen, no. 8101941, Lubeck, Germany) were utilized in the airplane pilot study to measure airborne concentrations between 5 and 100 p.p.grand. Measurements were taken at 2 ft above the spilled liquid and at adult breathing zone height (BZH) (5 ft) at selected fourth dimension periods later spilling a known amount of the cleaner product. The bathtub enclosure of bath three was utilized with the tub bleed airtight. During the spill study in this pocket-sized bathroom (about 320 ftiii), the bathroom fan (thirty cubic feet per minute, cfm) was operating, the entry door was open (no windows), and the shower enclosure sliding glass door was kept open up about 2 ft on one side for access. The shower door construction did not entirely enclose the bathtub expanse; in that location was 18 in of open infinite vertically betwixt the superlative of the door structure and the ceiling above the bathtub. Ambient indoor temperature was approximately 67°F. The "proper name brand" window cleaner spill was studied start, then the floor and tile cleaner spill was studied approximately two h later. Drager tube measurements prior to each spill event indicated no detectable airborne ammonia (<1 p.p.one thousand.).
The pilot spill study with the floor and tile cleaner was repeated two boosted times because most of the measurements in the pilot report had exceeded the calibration limit (5–100 p.p.m.) of the Drager tubes utilized. In subsequent experiments, a higher chapters (50–700 p.p.chiliad.) colorimetric tube (Drager-Rohrchen, no. 80020501, Lubeck, Germany) was utilized and measurements were made primarily at 5 ft to simulate the BZH of a typical developed. The container size and amount spilled were identical to the pilot study but were assessed in a unlike residence location. A small bathroom (560 ftthree) with standard bathtub enclosure was utilized, although there was no ventilation fan and a single window in the bathroom was opened at xv min subsequently each spill. Ambient indoor temperature was approximately 65°F. All measurements were made within the enclosure at two or v ft in a higher place the spilled liquid in the bathtub. All samples taken at ii ft above the liquid were nerveless to examine the ammonia vapor distribution with altitude above the spilled liquid, not to simulate whatsoever specific personal exposure situation.
Household Tile and Window Cleaning Exposure Studies
Personal exposures to ammonia were assessed for each production under use atmospheric condition as directed on the labels. Mixing of the floor and tile cleaner involved a continuing developed pouring the concentrate into a measuring cup placed on a countertop (well-nigh 30 in) to dispense one cup (8 ounces) of the cleaner immediately into a cleaning bucket (on the floor) containing either 1 or 2 gallons of hot water (approximately 105oF). The window cleaner (no mixing needed) was sprayed direct onto the windows liberally, such that larger droplets tended to condense and run downward the windows over nearly of the surface area existence cleaned.
Nosotros assessed instantaneous and issue-specific TWA exposures to airborne ammonia for a person performing household cleaning with the flooring and tile cleaner product in three tiled bathrooms of one house. Personal samples and area monitoring were conducted during mixing and cleaning equally described below. Exposure monitoring was conducted during mixing and utilize (cleaning with a soaked sponge), with less than 2 min to switch equipment between each of the bathrooms. All tiled and glass surfaces in each bathroom were done with the soaked sponge using the floor and tile cleaner. Each of the bathrooms contained a standard (30 cfm) frazzle fan that was in operation throughout the study, and the entry doors (all 3 rooms), shower/bathtub enclosure doors (all 3 rooms), and window (just for bathroom 2) were kept open during cleaning activities. Bath 1 was a minor bathroom (approximately 280 ft3) with a tiled floor, 24 in tiled vanity with two × iii foot mirror and fully tiled shower enclosure with glass door. Bath 2 was a big master bath (approximately 720 ftiii) with a tiled floor, big garden tub with tile surround upwardly to iv ft, a separate fully tiled shower enclosure with glass door, and a six ft double vanity with a 4 × 6 ft wall mirror. Bathroom three was another smaller bathroom (approximately 320 ftiii) with a tiled floor, xx in pedestal sink with 1.5 × three ft mirror and standard bathtub with a sliding drinking glass enclosure that was tiled from tub to ceiling. The tub in bath 3 was also used for the pilot spill scenario exposure study described above.
Airborne ammonia concentration versus fourth dimension profiles were also assessed for a diversity of cleaning scenarios with diluted or total strength (concentrate) flooring and tile cleaner in a separate residence. This included cleaning and scrubbing the tile floor in two bathrooms and a den area, equally well as employ of poured concentrate on countertops in the kitchen and bathrooms, and use of concentrate inside the bathtub enclosures. The volume of concentrate used in each cleaning scenario was approximately iv tablespoons or capfuls (one capful=1 tablespoon), where the person cleaning quickly measured out iv capfuls, poured it on the countertop and spread it evenly using dry paper towels. Bath 4 was approximately 560 ft3 with a 70 fttwo tiled floor, tiled double vanity (12 ftii), and tiled bathtub enclosure with drinking glass entry door that had an xviii in vertical infinite above the glass enclosure. Bathroom 5 was approximately 470 ft3 with a 59 ft2 tiled flooring, tiled double vanity (10 ft2), and tiled bathtub with glass entry door with an 18 in vertical infinite higher up the glass enclosure. The bathroom doors and bathtub enclosure doors were left open during each cleaning written report. The den was a tiled expanse within a larger open expanse of the business firm with a volume of approximately 4600 ft3. The kitchen area had approximately 21 ftii of countertop expanse that was cleaned within a 1600 ft3 book that was open up on one side to the den area. In this residence, at that place was no active ventilation with outdoor air from windows, exterior doors, fans, or air conditioning devices until the cleaning steps were completed, as noted in the figures.
We also assessed exposures during utilise of the "name make" spray-on drinking glass cleaner, while washing several big windows in an function setting. Standard office building air conditioning was operating during the written report. Seven big panel windows (approximately five × 5 ft) were washed during the study, one panel at a fourth dimension, using paper towels to wipe and dry each panel. Four of the window panels were contained in one large role (approximately 2600 ft3) and the remaining three panels were contained in an side by side office (approximately 1600 ftthree). Similar window cleaning procedures were completed in a different office building using the "brand proper name" window cleaner and separately using a "store brand" window cleaner using the PAC-III monitor only. The book of product used during spraying of similar square footage of window space was determined by weighing the containers before and subsequently the cleaning for three iterations.
Airborne Ammonia Measurement Methods
For the tile and window cleaning studies, two unlike measurement methods were utilized: NIOSH Method 6015 and the Drager PAC(Three) field monitor. The first is a laboratory-based method that involved collection of air at BZH using a calibrated, bombardment-operated pump (SKC Universal, model 224-PCXR4, Valley View, PA, USA) that pulled air through a sorbent tube (SKC detail no. 226-10-06, 6 × 60 mm size, 2-secction, 100/200 mg silica gel). Each tube was labeled and sent with chain of custody records to an American Industrial Hygiene Association (AIHA) certified laboratory (DataChem Laboratories, Salt Lake City, UT, United states) that extracted the sample and measured ammonia levels in accordance with NIOSH Method 6015. In the first set of bath tile cleaning studies (bathrooms 1, 2, and 3), the person performing the cleaning activities (MJF) had one BZH monitor on his collar/lapel (in add-on to the PAC-Three monitor) while cleaning all 3 bathrooms. Concurrent expanse samples located in the centre of each bathroom at BZH were collected but during the cleaning activities in that room, and were analyzed past NIOSH Method 6015. In the second series of measurements in a separate residence (bathrooms iv and five, den and kitchen), the PAC-III monitor was again worn on the collar or lapel of the person performing the mixing and cleaning tasks, simply no surface area samples were collected.
The Drager PAC Iii monitor was equipped with an electrochemical cell detector (DragerSensor XS EC NH3-68 09 145, Drager, Germany) that was pre- and post-calibrated for ammonia. The PAC(III) monitor samples a continuous stream of air and records a measurement every lx s. The monitor was calibrated to measure airborne ammonia levels upward to 200 p.p.chiliad. with a reliable detection limit of 3 p.p.one thousand. identified by the manufacturer. Instrument scale was checked by the industrial hygiene supply business firm before and after the two series of measurements and no notable deviations were identified (< 5% variance between the pre- and post-exam calibration check, data not shown).
Results
Cleaner Production Ammonia Content
Tabular array ane provides a summary of the measurements for pH and ammonia content of the three cleaner products examined in these studies. The flooring and tile cleaner, which on the label and material safety data sheet (MSDS) was said to be a v% ammonia solution, was found to contain virtually 3% ammonia (wt/vol). The "name brand" glass cleaner, said to contain between 1% and three% ammonia on the 2001 MSDS, was found to contain only well-nigh 0.1% ammonia. A 2004 MSDS for the aforementioned name make product did not indicate the ammonia content. A like glass cleaner that was a "store brand" production was found to contain approximately 0.05% ammonia and as well did not list the ammonia content on their 2004 MSDS. 2 of the three samples were analyzed in duplicate assays and institute to be in close agreement (<5% variance, see footnote of Table 1). The pH of all 3 tested products was quite bones, with both glass cleaners at effectually pH 10.9 and the flooring and tile cleaner at pH 11.7, indicating that essentially all of the ammonia in solution was likely in the grade of dissolved NH3, non ammonium ion (NHfour +). Testing of the diluted floor and tile cleaner indicated like basic pH (approx. pH x with litmus paper) to that shown for the fix-to-use glass cleaners in Tabular array i (data not shown).
Spill Exposure Studies
Table two provides a summary of airborne ammonia concentrations measured in the tub enclosure of bath iii following spillage of about one-half of each container of the floor and tile cleaner and the spray-on window cleaner; weights before and afterward the fake spills corresponded with averaged spilled volumes of 33.ane. and 17.3 ounces, respectively (three trials each container, data not shown). Ammonia concentrations in the pilot report frequently exceeded the upper scale limit of the colorimetric tubes for the floor and tile cleaner. In repeated trials using higher capacity colorimetric tubes, ammonia volatilization from the floor and tile cleaner product led to airborne concentrations consistently exceeding 200 p.p.m. for 20–xxx min after the spill, despite the bathroom window and door being opened later xv min. Table ii besides illustrates much lower airborne ammonia levels later on spilling nigh half the container (17 ounces) of the spray-on window cleaner. Ammonia levels measured at ii-ft height showed a consistent airborne concentration of vii–8 p.p.grand. at between 4 and x min after the spill, while measurements at BZH (5-ft) were nearly one-half those observed at ii ft during the same time menses.
The ammonia concentrations above the spilled name brand spray-on product solution were lower than would exist expected based on the book spilled and the stated pct ammonia content (1–3% × 17 ounces) in comparison to the flooring and tile cleaner (5% × 33 ounces). However, the measured production ammonia content was lower than that stated. For example, linear adjustment of the spray-on window cleaner data at BZH (eastward.g., four p.p.one thousand. at iv–10 min) for the difference in measured percent ammonia (times 3/0.i%) and volume spilled (times 33.1 ounces/17.3 ounces) leads to an expected exposure concentration of about 230 p.p.m. above the spilled flooring and tile cleaner. Instead, airborne ammonia concentrations under these conditions rose to >450 p.p.m. to a higher place the spilled floor and tile cleaner product.
Exposures Assessed During Label-Directed Product Uses of Floor and Tile Cleaner
Standard Force Cleaning Solution: 0.1% Ammonia
The airborne ammonia sampling data for label-directed use of the floor and tile cleaner at standard cleaning strength (one cup per 2 gallons) are summarized in Figure one. The timing of specific activities and cleaning events in the three bathrooms are summarized in the footnote to Effigy i. Height ammonia levels at BZH measured with the PAC Iii monitor (personal sample) occurred within 2–iii min subsequently mixing a bucket full (2 gallons) of the floor and tile cleaner solution in the outset and smallest bathroom (#1), whereas the other ii larger bathrooms had acme levels occurring later about 5 min of cleaning activity using the same bucket of cleaner without further mixing events (Figure i). Bathrooms 2 and 3 had elevation exposures recorded during cleaning in the shower or tub enclosure, while bathroom 1 had peaks apparently associated with mixing the cleaner solution and after entry and cleaning in the shower enclosure. Peak exposure concentrations approached, but did not exceed the ACGIH 8-h TWA occupational exposure limit of 25 p.p.m., or the ACGIH and NIOSH-recommended short term exposure limit of 35 p.p.m., which is a more appropriate comparison signal for these curt-term (<10 min) peak exposure measurements.
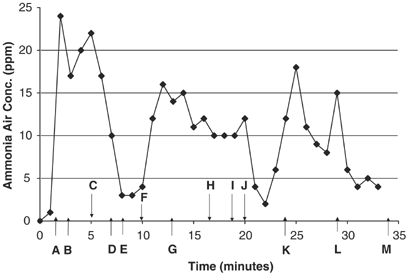
Airborne ammonia concentrations in three bathrooms during utilize of diluted flooring and tile cleaner (0.1% ammonia), PAC-III Lapel Monitor. Letters denote the start time of the following events: (A) mixed 1 loving cup floor and tile cleaner with two gallons hot water in a saucepan inside bathroom 1; (B) brainstorm cleaning tile flooring, vanity counter, sink, and small mirror of bathroom 1 with soaked sponge; (C) brainstorm cleaning shower enclosure tile walls, floor and drinking glass door; (D) end cleaning of bathroom 1 and transport equipment to bathroom 2; (E) ready equipment in bathroom 2; (F) brainstorm cleaning tile flooring in bathroom 2 with soaked sponge; (G) brainstorm sponge cleaning of shower enclosure tile wall, floor and glass door, countertop, large vanity mirror, and tile surround and bathtub; (H) end cleaning bathroom 2 and transport equipment to bath 3; (I) gear up up equipment in bathroom 3; (J) begin cleaning tile floor in bath 3 with soaked sponge; (One thousand) begin cleaning bathtub, surrounding tile and glass doors on tub enclosure; (50) brainstorm cleaning vanity sink and minor mirror; (M) stop of cleaning in bathroom 3.
Airborne ammonia levels based on area samples taken in the centre of each bathroom at BZH (NIOSH 6015) are summarized in Table 3. The calculated TWA for these 3 area samples was 9.1 p.p.1000. during cleaning activities, whereas the personal sample (also NIOSH 6015) collected during all the bathroom cleaning activities showed x p.p.thou. Thus, there is skillful agreement between the area samples and personal sample ammonia levels adamant using NIOSH Method 6015.
Table iii also shows good agreement between the concurrent measurements utilizing NIOSH Method 6015 and the PAC Iii monitor, both of which were mounted to the neckband/lapel of the person conducting the cleaning. The PAC III monitoring data (readings taken every lx s) were averaged across the appropriate outcome-specific sampling periods to arrive at TWAs for comparison with the NIOSH Method 6015 information. Calculated values for the PAC 3 monitoring data show slightly higher boilerplate levels compared to the concurrent NIOSH Method 6015 results for the floor and tile cleaning activities (Table iii).
Double-Strength Cleaning Solution: 0.2% Ammonia
Airborne ammonia profiles for bathroom tile cleaning in a separate home using double the standard strength cleaning solution are illustrated in Figure 2. Bathroom 4 and bath v were cleaned with the flooring and tile cleaner solution at 0.two% ammonia content (1 cup per gallon). Both bathrooms had no active ventilation during the mixing and cleaning steps, although the entry doors remained open at all times. The cleaning steps included mopping the flooring with a sponge-type mop and scrubbing spots on the floor with cleaner-soaked paper towels, but no cleaning was done of the vanity countertops or within the bathtub enclosure. Superlative exposure during initial mopping reached about xc p.p.thousand. in both bathrooms at iii–v min later mixing the cleaner solution with continuous cleaning thereafter. Scrubbing with soaked paper towels on hands and knees did increase the measured ammonia exposure concentration as expected, only overall height exposures seemed to correlate best with time since the cleaning began (and probably the total volume of ammonia cleaner spread over the surfaces cleaned). The boilerplate exposure concentration during cleaning was approximately 13–35 p.p.m. for the cleaning steps (time cypher to end of cleaning) and 23 p.p.chiliad. for the cleaning and ventilation steps (from time zero until the monitor returned to 0 p.p.m. after cleaning). Figure 2 also illustrates the rapid reject in airborne ammonia concentrations following the 0.2% cleaner usage, taking only ii–3 min to turn down from 62 p.p.k.–xi p.p.m. in a bathroom with no forced ventilation other than opening the window (the entry door was open at all times).
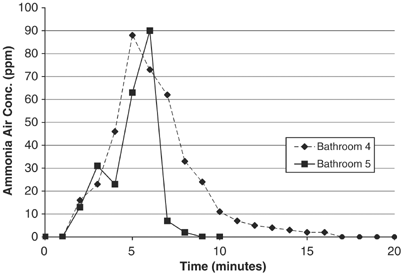
Airborne ammonia concentrations during bath tile cleaning with diluted flooring and tile cleaner (0.2% ammonia), PAC-Iii Lapel Monitor. Relevant activities in bathroom 4: (T=i.5) mixed 1 cup floor and tile cleaner with ane gallon hot water in a saucepan within bath 4; (T=3) soak sponge mop, wring lightly, and begin mopping the tile floor; (T=5) scrub the tile floor with soaked newspaper towels while on hands and knees; (T=8.25) terminate of cleaning, open window in bathroom to vent; and in bath 5: (T=one) mixed 1 cup floor and tile cleaner with 1 gallon hot water in a saucepan inside bathroom 5; (T=2) scrub the tile floor with soaked paper towels while on easily and knees; (T=3) soak sponge mop, wring lightly, and brainstorm mopping the tile flooring; (T=6) stop of cleaning in bathroom 5; (T=vi.25) remove monitor to outdoors to clear sensor.
Effigy 3 presents the airborne ammonia profile for tile cleaning with the 0.2% ammonia solution in an open den surface area of the same home. A larger floor surface area, approximately 100 ftii of tile, was cleaned using both mopping while continuing and scrubbing with soaked paper towels while on hands and knees. The pinnacle ammonia level at 36 p.p.one thousand. occurred inside 2.5 min afterwards mixing and so mopping the unabridged area once (at fourth dimension=two–four min). Scrubbing the floor on hands and knees at time=4–6 min did not atomic number 82 to increasing exposure concentrations, again suggesting that total cleaner book dispensed may be a better indicator of exposure potential than distance between the cleaning surface and one's animate zone. The average exposure during cleaning (0–7 min) was nearly 16 p.p.m. and for cleaning and ventilation to nondetectable levels (0–17 min, inclusive) was approximately 11 p.p.m. In this scenario, a large sliding glass door was opened at time=vii min (open area well-nigh 5 × 7 ft). The ammonia vapors dissipated more slowly compared to the bathrooms (Figure ii) where the floor surface cleaned (and cleaner volumes dispersed) were much lower. There was no forced ventilation in the den area other than opening the sliding glass door.
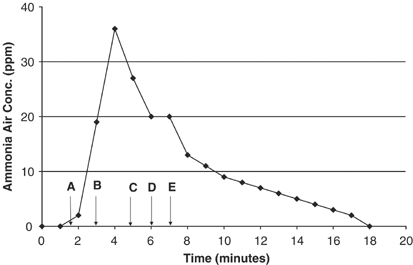
Airborne ammonia concentrations during den tile cleaning (100 fttwo) with diluted flooring and tile cleaner (0.2% ammonia), PAC-III Lapel Monitor. Letters denote the start time of the following events: (a) mixed 1 cup of floor and tile cleaner with 1 gallon hot water in a bucket in den area; (b) soak sponge mop, wring lightly, and begin mopping tile floor; (c) scrub the tile floor with soaked newspaper towels while on easily and knees; (d) cease of cleaning in den surface area, bucket and soaked towels were left in the cleaned surface area; (e) opened sliding glass door to vent, but monitor remained in cleaned area at BZH.
Total Strength Concentrate Cleaner Usage: 3% Ammonia
Figure 4 presents the airborne ammonia profile for use of a small book (4 tablespoons) of the total forcefulness floor and tile cleaner to clean the countertops in bathroom 4. The summit ammonia level at 200 p.p.thou. occurred within 3 min later pouring four tablespoons of the concentrate onto a 12 ft2 countertop and immediately spreading it with dry paper towels. The summit ammonia levels in the bathroom space declined rapidly after the bathroom window was opened at time=v.5 min. The average exposure during cleaning steps (about 0–4 min) was well-nigh 63 p.p.m. and for the entire flow monitored (0–sixteen min) was virtually 33 p.p.m.
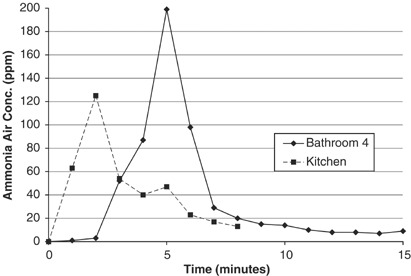
Airborne ammonia concentrations during bath countertop cleaning with concentrated floor and tile cleaner (3% ammonia), PAC-3 Lapel Monitor. Relevant activities in bath iv: (T=2) pour iv tablespoons of floor and tile cleaner on countertop and spread with dry paper towels; (T=iv) end of cleaning in bathroom but remained in forepart of vanity and placed the used paper towels in an open-summit bathroom garbage tin can; (T=5.5) opened window in bath; (T=13.v) monitor placed outdoors to articulate sensor; and in the kitchen: (T=0.25) poured 4 tablespoons of undiluted floor and tile cleaner on countertop and spread with dry paper towels; (T=2.25) completed wiping, placed the used paper towels in kitchen garbage tin can within a cabinet under the kitchen sink; (T=3.5) continued standing in front of cleaned countertop; and (T=iv.5) monitor placed outside to clear the sensor.
Figure 4 likewise presents a countertop cleaning scenario with 4 tablespoons of the concentrate used on the 21 ft2 kitchen countertop. The countertop area and the room volume were much larger for the kitchen compared to bathroom iv, and the kitchen was open to the den area. The tiptop ammonia concentration of 125 p.p.m. occurred within 2 min after pouring the concentrate onto the countertop and immediately spreading it to sanitize the surfaces. The average exposure concentration was about 56 p.p.m. during the cleaning steps (0–4 min).
Two additional studies were attempted to examine ammonia exposure concentrations for cleaning with 4 tablespoons of the three% solution on soaked newspaper towels inside the tub enclosures of bathrooms 4 and five with the enclosure door left open. In each case, the PAC-3 monitor detected apace ascension concentrations (increasing by 50–80 p.p.m. per minute) that exceeded 200 p.p.1000. inside ii.five min after the tile cleaning inside the tub enclosure began (data not shown). In both trials, irritation of eyes and nasal passages was intolerable to the cleaner once ammonia concentrations approached 200 p.p.m. and hence the full concentration versus time profiles were not obtained.
Exposures During Label-Directed Use of the Name Brand Glass Cleaner Product
Airborne ammonia concentrations measured during the window cleaning activeness using the spray-on glass cleaner were considerably lower than those for use of the floor and tile cleaner at comparable ammonia strength (0.1%. solution). The book of cleaner used in this cleaning scenario was approximately 0.3 ml per square foot of drinking glass cleaned. The PAC 3 monitoring data varied between 0 and ii p.p.yard. during window cleaning activeness, only these recorded levels are below the musical instrument manufacturer's reliable detection limit of 3 p.p.m. (data not shown). Table 3 shows that the personal sample analyzed co-ordinate to NIOSH Method 6015 showed an exposure concentration of 0.65 p.p.g. for the window cleaning activity. The concurrent data from the PAC III monitor averaged 1.1 p.p.thou., which despite detection limit issues tends to reinforce the relatively low exposure levels associated with a typical use of this window cleaner product. Similar use of the store brand glass cleaner (0.05%) led to low airborne ammonia exposures also (0–two p.p.1000. using the PAC-3 lapel monitor, data not shown). The floor and tile cleaner product used at 0.one% force led to virtually xiv-fold higher TWA exposures than for utilize of the name brand window cleaner at similar ammonia force (NIOSH Method 6015 results, Table 3), This differential must be due in big part to the much smaller volume of solution dispensed with the window cleaner pump sprayer.
Discussion
This study provides information on the pattern and magnitude of airborne ammonia concentrations from limited spill scenarios and for selected label-directed household cleaning uses of two common types of ammonia-containing products in diverse indoor settings. No similar cleaning exposure studies or data sets were identified in our searches of the published literature to date. Our data indicate distinct differences in exposure potential between ii spray-on window cleaner products at gear up-to-use concentrations and a household floor and tile cleaner product with label-recommended uses for full force and various diluted cleaning solutions.
A recent written report past Webb et al. (2002) reported on ammonia concentrations in a room-size study bedroom (1720 ft3) in which they assessed nicotine desorption from furniture and wall surfaces exposed to environmental tobacco fume when a bucket of household ammonia cleaner (2 cups in ane gallon of hot water) was placed inside. The dissolved ammonia concentration was not reported, simply would correspond to near 0.4% if their household cleaner had 3% ammonia similar the product we studied. They reported a peak concentration of 30 p.p.m. airborne ammonia at 2 h after placing the bucket inside the room. This was a vapor equilibrium study, not a cleaning study. Although the experimental weather are quite different from the electric current written report, their tiptop ammonia level of 30 p.p.m. may be compared with our tiptop of 36 p.p.thou. for the den tile floor cleaning scenario with a 0.two% ammonia solution.
The current report findings indicate that the potential for excessive consumer exposures to airborne ammonia is much greater for the household floor and tile cleaner product during accidental spills or in expected uses involving poorly ventilated spaces or use of stronger cleaning solutions than the standard dilution (0.one%) directed on the product label. In contrast, the window cleaner product did not demonstrate potential for excessive airborne ammonia exposures in the simulated accidental spill or expected apply scenarios. While the room size and air commutation rate were very unlike for the expected use scenarios between the two products, the spill study data conspicuously bear witness a far greater exposure potential with the floor and tile cleaner production.
In that location are several uncertainties and caveats that should be noted with respect to these data. First, we did non attempt to fully characterize the indoor environment conditions and other cleaner volume parameters that might be useful to accurately model indoor ammonia concentrations. Nosotros did not assess mass balance, airflow characteristics or humidity, and did not attempt to measure the book of cleaner used in most of the cleaning scenarios presented. However, we believe that the cleaning scenarios in the current study are representative of mutual household usages. Thus, the application of these data to other situations should consider the limitations of this study. Second, we discovered that the ammonia content data listed on a container or on the MSDS may not be accurate, and may only point an upper spring content. Products similar the window cleaners tested here are only required to list ammonia content on the product MSDS if it exceeds one% (wt/vol). Plus, the MSDS does not accept to list the precise formula or content of ammonia; rather, information technology must list a non-to-exceed level or range if the final formulated product could contain >1% ammonia. Third, nosotros encountered issues with determining the production ammonia content when utilizing a 2nd USEPA-certified laboratory that was non certified for consumer product and pharmaceutical analyses (Skillful Manufacturing Practices Certification). Results obtained using the Full Kjeldahl Nitrogen method in the second laboratory were like for the concentrate floor and tile cleaner (31,000 mg/l). All the same, the gear up-to-utilize glass cleaners were reported to incorporate 2–four-fold higher concentrations than expected based on the other laboratory, and at that place were analytical bug encountered in digesting the diluted samples. Thus, we relied solely on the other laboratory'due south results (Table i) that had good quality control information.
Review of Dose–Response considerations
Attributable to the natural presence of ammonia in sewage, fertilizers, animal excreta, and other processes there is a constant groundwork exposure to airborne ammonia in the ambient air. Several researchers have examined background air concentrations not influenced by local sources and adamant that low part per billion (p.p.b.) ammonia levels, for instance, 0.1–5 p.p.b., are common in both urban and rural areas throughout the world (Georgii and Gravenhorst, 1977; Farmer and Dawson, 1982; Harward et al., 1982; Tanner, 1982; Crutzen, 1983; Dawson and Farmer, 1984; Kelly et al., 1984; Russell et al., 1988; Fangmeier et al., 1994; Aneja et al., 1998). Similar levels have been reported for airborne ammonia concentrations over the world's oceans (Georgii and Gravenhorst, 1977).
Controlled ammonia exposure studies take shown sensory irritation in unadapted individuals exposed at 20–thirty p.p.one thousand. for ten–15 min (Vigliani and Zurlo, 1956; MacEwen et al., 1970; Industrial Bio-Test Laboratories, 1973; Verberk, 1977; Sekizawa and Tsubone, 1994); withal, tolerance is commonly observed with repeated exposures (Verberk, 1977; Sekizawa and Tsubone, 1994). Other astute exposure studies in humans further demonstrate moderate to astringent respiratory and ocular irritation from airborne ammonia exposures at or above 50 p.p.1000. Silverman et al. (1949) exposed volunteers to 500 p.p.grand. ammonia for 1.5 h and observed lacrimation, nasal, and throat irritation, and increased minute volume and respiratory rate. Industrial Bio-Test (1973) reported on volunteers exposed to ammonia vapors for 5 min and observed nasal and throat irritation at 72 p.p.m., just not at 50 p.p.m. Cole et al. (1977) exposed male volunteers to ammonia vapors for eight–eleven min while exercising and observed decreased minute book and increased tidal volume at 150–331 p.p.m., simply not at 100 p.p.k. Verberk (1977) exposed volunteers to l p.p.m. ammonia vapors for ane.5 h and observed nose and throat irritation, urge to cough, and middle irritation.
Longer-term studies reveal a design of tolerance to irritation symptoms and no demonstrated pulmonary office deficits for ammonia exposures below 25 p.p.grand. Ferguson et al. (1977) reported a written report of intermediate exposure duration for volunteers exposed at l or 100 p.p.yard. ammonia for 6 weeks (6 h per mean solar day, 5 days per week) and reported transient centre, olfactory organ, and throat irritation at 100 p.p.g. but not at 25 or 50 p.p.m.; these exposures were not associated with any clinical changes in lung office tests, physical exam findings, or performance of normal job duties. Holness et al. (1989) reported that chronic occupational exposure to average levels of 12.5 p.p.one thousand. ammonia in a grouping of soda ash factory workers did not cause clinical changes in pulmonary function tests or odor sensitivity. Ballal et al. (1998) reported a cantankerous-sectional study of asthma and respiratory complaints among male workers at ii fertilizer factories. They reported that workers exposed to ammonia levels by and large in a higher place 25 p.p.g. had significantly higher risks of respiratory symptoms and asthma, particularly for those in Manufactory 1 where airborne ammonia levels ranged from nigh 3 to 184 p.p.grand. Workers in Factory 2 where ammonia exposure levels were always beneath 25 p.p.m. (range 0.03–10 p.p.thousand.) reportedly did not have significantly elevated risks for respiratory symptoms or asthma.
ATSDR (2002) adult a minimal risk level (MRL) for astute inhalation exposure at 1.vii p.p.m. based on the Verberk (1977) study showing mild irritation in humans at 50 p.p.m. × 2 h, and a chronic inhalation MRL at 0.3 p.p.k., based on the soda ash worker study by Holness et al. (1989) showing no respiratory effects after chronic occupational exposures (boilerplate xiv years employment) averaging 12.5 p.p.m. The United states of america Ecology Protection Agency (USEPA; IRIS, 2003) derived a similar chronic standard for ammonia inhalation called the reference concentration (RfC) at 0.1 mg/g3, equivalent to most 0.14 p.p.grand. Both the MRL and RfC values are developed to apply to assumed continuous exposures to the general population, and contain various modifying factors and uncertainty factors to account for potentially sensitive individuals. However, these concentrations are well below those that take been demonstrated to take adverse health furnishings in humans.
Recommended workplace exposure limits are based on assumed exposure to healthy workers for 8–ten h per day, and hence are less bourgeois than the general population safe exposure estimates (MRL and RfC). Although the OSHA permissible exposure limit (PEL) remains at 50 p.p.m. every bit an 8-h TWA, the NIOSH recommended exposure limit and the ACGIH (2001) threshold limit value are set at 25 p.p.m. as an eight-h TWA. The short-term exposure limit (xv-min TWA) recommended past NIOSH and ACGIH is 35 p.p.thousand. ammonia.
Production-Specific Hazard Assessment
Every bit illustrated in the spill exposure study, a bathroom spill of about 33 oz of 3% ammonia may produce inhalation exposures as loftier as 550 p.p.m. airborne ammonia, even with window ventilation and the bathroom door open. These exposures far exceed the electric current occupational and environmental condom limits for acute inhalation exposure including the ACGIH and NIOSH brusque-term exposure limit of 35 p.p.one thousand. (15-min average), the ATSDR minimal adventure level for acute exposures (1.7 p.p.m.), and the two virtually restrictive AIHA emergency response planning guidelines (ERPG-1=25 p.p.g. and ERPG-2=200 p.p.m.). Airborne ammonia levels later such a spill exceeded 200 p.p.one thousand. for at least xv–25 min after the spill inside a bathtub/shower enclosure. Extended exposures at concentrations in a higher place 300 p.p.yard. could cause significant respiratory tract and ocular irritation in normal adults or children, but may pose more than severe risks to persons with asthma or sure other pulmonary diseases. However, these exposures involving spills and usage of 3% ammonia in household settings more often than not dissipate apace enough to avoid clinically of import corrosive damage to the lungs as is observed with exposures >5000 p.p.k. fifty-fifty for a matter of minutes. Overall, the warning properties of ammonia should be sufficient to induce avoidance actions in almost situations, unless egress was impeded post-obit a spill.
In contrast, the spill exposure study for the spray-on window cleaner production showed height exposures at BZH that were below 5 p.p.m. This occurred despite the finding that the high pH (10.nine) of glass cleaner solutions indicates the vast majority of dissolved ammonia was in the grade of volatile NH3 gas, not ammonium ion. In the product spill scenario, information technology seems unlikely that the spray-on window cleaner would lead to chancy airborne ammonia levels. The airborne ammonia exposures during the window cleaner spill were lower than expected; we did not specifically investigate this effect, merely speculate that other surfactants or solvents (ethylene glycol northward-hexyl ether, and isopropanol) added to the window cleaner may impede ammonia vapor release from the aqueous solution. Moreover, normal employ of this set up-to-use aerosol pump product would not require removal of the cap/pump machinery except mayhap for refilling.
The label-directed uses of the floor and tile cleaner production led to short-term exposures that for the standard cleaning solution strength (1 cup per two gallons or well-nigh 0.1%) averaged effectually 10 p.p.m. airborne ammonia and showed superlative values betwixt 16 and 24 p.p.m. These peak exposure levels arroyo merely practice not exceed the ACGIH/NIOSH short-term exposure limit of 35 p.p.m., and the average exposure level in ventilated bathrooms was less than one-half of the NIOSH and ACGIH occupational exposure limit recommendation of 25 p.p.m. as an 8-h TWA. Yet, subsequent studies using the 0.2% ammonia solution led to superlative exposures of around 90 p.p.grand. in ii minor, unventilated bathrooms and 36 p.p.1000. in a more open den surface area while mopping and scrubbing tile floors. These product usage conditions can readily lead to peak and boilerplate exposures to consumers at or higher up the recommended occupational short-term exposure limits, but misemploy relatively rapidly over the post-obit 10–15 min without forced ventilation. Likewise, as noted above, individuals with potentially greater sensitivity to respiratory effects from irritant exposures, for example, asthmatics, may nonetheless feel acute respiratory problems even under these peak exposure concentrations (e.g., 36–90 p.p.m.).
We too examined exposures for utilise of the concentrate (undiluted) floor and tile cleaner for sanitizing countertops or the tile within tub enclosures. Use of only a few tablespoons of the undiluted cleaner on countertops in an open kitchen area or in a small bathroom led to peak ammonia concentrations betwixt 100 and 200 p.p.m. within one–2 min after dispensing the product. Again, the airborne ammonia levels dissipated within a few minutes, except in more than independent spaces similar a bathtub enclosure. Thus, using the concentrate (3% solution) in large volumes and/or in contained spaces can lead to exposures exceeding the ERPG-2 guideline of 200 p.p.k.
The label-directed use of the spray-on window cleaner product led to much lower short-term ammonia exposures (about 0.seven p.p.one thousand.) compared to the flooring and tile cleaner at 0.one% (average 10 p.p.grand.) and 0.2% solutions (boilerplate about 11–30 p.p.m.). The intentional generation of coarse spray aerosols past the mechanical pump dispenser did not lead to substantial exposures (<two p.p.m.). Indeed, the pump mechanism appears to simultaneously limit the full volume of cleaner (including ammonia) dispensed, and prevent appreciable vaporization losses of ammonia remaining in the bottle of cleaning solution. Appropriately, at that place appears to be no substantial inhalation hazard associated with normal cleaning uses of the ammonia-containing spray-on window cleaner production.
Our data on exposure and take chances potential for the products under written report correlate with the medical consequences of domestic users reported in a contempo compilation of data from Us poisonous substance control centers (Watson et al., 2003). In 2002, at that place were 2418 reported exposures to household ammonia cleaners (e.chiliad., flooring and tile cleaners) with 437 (18.ane%) resulting in treatment at a health care facility and 163 of those (163/437 or 37.3%) with moderate to major outcomes. For household glass cleaners with ammonia, 1564 exposures were reported with 127 (viii.1%) treated in wellness intendance facilities and merely 20 (fifteen.7%) with moderate outcomes. No deaths were reported from either type of household ammonia exposure, but children under age six years comprised 78% of reported exposures involving drinking glass cleaner and only forty% of exposures involving ammonia cleaners. These statistics exercise not distinguish inhalation exposures from ingestion exposures that are specially common in immature children. Inhalation exposures from all fumes, gases, or vapors comprised about 3.5% of all poison command eye calls in 2002, simply those attributable to ammonia were not delineated. Nazaroff and Weschler (2004) notation that many poisoning incidents may involve reaction products of improperly combining household cleaners, as in chloramine gas generation from combining household ammonia and bleach. They also reported that at least 26 case reports implicate cleaning or cleaning products every bit the cause of respiratory impairment, although simply i of the cited reports involved ammonia vapors every bit the suspect toxic agent.
Most consumers would be expected to accept protective actions to avoid ammonia exposures when atmospheric levels produce sensory irritation, for instance, at exposure concentrations higher up 25–50 p.p.m. Significant respiratory irritation from spills or usage of the household ammonia cleaner studied hither can be avoided by assuring that adequate ventilation is used and/or that persons are evacuated from the impacted area until the emission source has been largely exhausted. Persons with pre-existing respiratory conditions such equally asthma or other obstructive lung diseases are more often than not considered to exist at increased gamble of adverse health consequences from exposure to atmospheric irritants like ammonia. The short-term exposure limits for workplace ammonia exposures (35 p.p.m.) may non be sufficiently depression to protect these individuals from experiencing respiratory effects during spillage and certain uses of concentrated household ammonia products like the floor and tile cleaner examined in the current report.
References
-
ACGIH (American Conference of Governmental Industrial Hygienists). Ammonia. CAS number 7664-41-7, 2001.
-
AIHA (American Industrial Hygiene Association). Emergency Response Planning Guidelines. Ammonia, Fairfax, VA, 2001.
-
Aneja V.P., Murthy A.B., Battye W., Battye R., and Benjey W.1000. Assay of ammonia and aerosol concentrations and deposition virtually the costless troposphere at Mt. Mitchell, NC, USA. Atmos Environ 1998: 32 (3): 353–358.
-
ATSDR (Bureau for Toxic Substances and Disease Registry). DRAFT Toxicological Profile for Ammonia. US Department of Health and Homo Services, Public Wellness Service, 2002.
-
Ballal South.K., Ali B.A., Albar A.A., Ahmed H.O., and al-Hasan A.Y. Bronchial asthma in two chemical fertilizer producing factories in eastern Saudi Arabia. Int J Tuberc Lung Dis 1998: ii (4): 330–335.
-
Bradstrut R.B The Kjeldahl Method for Organic Nitrogen. Academic Press, New York, 1965.
-
Cole T.J., Cotes J.E., Johnson G.R., Martin H.D., Reed J.W., and Saunder J.E. Ventilation, cardiac frequency and pattern of breathing during exercise in men exposed to o-chlorobenzylidene malonitrile (CS) and ammonia gas in depression concentrations. Q J Exp Physiol Cogn Med Sci 1977: 63: 341–351.
-
Crutzen P.J. Atmospheric interactions—homogeneous gas reactions of C Northward, and South containing compounds. In: Bolin B., and Melt R.B. (Eds.). The Major Biogeochemical Cycles and their Interactions. John Wiley & Sons, Chichester, 1983, pp. 67–113.
-
Dawson K.A., and Farmer J.C. Highly soluble atmospheric trace gases in the Southwestern United States. I. Inorganic Species: NHiii, HNO3, Theniii. J Geophys Res 1984: 89 (D3): 4779–4787.
-
Fangmeier A., Hadwiger-Fangmeier A., Van Der Eerden L., and Jager H.J. Effects of atmospheric ammonia on vegetation: a review. Environ Pollut 1994: 86 (1): 43–82.
-
Farmer J.C., and Dawson Chiliad.A. Condensation sampling of soluble atmospheric trace gases. J Geophys Res 1982: 87 (C11): 8931–8942.
-
Ferguson Due west.S., Koch Due west.C., Webster L.B., and Gould J.R. Human physiological response and adaption to ammonia. J Occup Med 1977: nineteen: 319–326.
-
Georgii H.W., and Gravenhorst G. The bounding main every bit source or sink of reactive trace-gases. Pure Appl Geophys 1977: 115: 503–511.
-
Harward C.N., McClenny Westward.A., Hoell J.Chiliad., Williams J.A., and Williams B.S. Ambience ammonia measurements in coastal Southeastern Virginia. Atmos Environ 1982: 16 (ten): 2497–2500.
-
Holness D.Fifty., Purdham J.T., and Nethercott J.R. Acute and chronic respiratory effects of occupational exposure to ammonia. Am Ind Hyg Assoc J 1989: l (12): 646–650.
-
Industrial Bio-Test Laboratories, Inc. 1973. Irritation threshold evaluation report with ammonia. Report to International Constitute of Ammonia Refrigeration (IBT No. 663-03160), Northbrook, IL; March 23, 1973 Industrial Bio-Test Laboratories, Inc. [unpublished study to be peer reviewed].
-
Integrated Chance Information System (IRIS). Ammonia, Us Environmental Protection Bureau, 2003.
-
Kelly T.J., Tanner R.50., Newman L., Galvin P.J., and Kadlecek J.A. Trace gas and aerosol measurements at a remote site in the Northeast The states. Atmos Environ 1984: xviii (12): 2565–2576.
-
MacEwen J.D., Theodore J., and Vernot Eastward.H. Homo exposure to EEL concentrations of monomethylhydrazine. Proceedings of 1st Almanac Conference on Environmental Toxicology, AMRL-TR-70-102, Newspaper 23, September 1970; Wright-Patterson Air Forcefulness Base, Dayton, Ohio, 1970.
-
National Academy of Sciences. Guideline for Short Term Exposures of the Public to Air Pollutants. Four. Guide for Ammonia past the National Inquiry Council, Committee on Toxicology. National Academy Printing, Washington, DC, 1987.
-
Nazaroff W.West., and Weschler C.J. Cleaning products and air fresheners: exposure to principal and secondary air pollutants. Atmos Environ 2004: 38 (4): 2841–2865.
-
NIOSH (National Found for Occupational Safety and Health). Criteria for a Recommended Standard – Occupational Exposure to Ammonia. DHEW Pub. No. 74-136, DHHS Pub. No. 97-106, NTIS No. Lead-502-082, 1997.
-
Russell A.G., McCue K.F., and Cass G.R. Mathematical modeling of the formation of nitrogen-containing air pollutants. 1. Evaluation of an Eulerian photochemical model. Environ Sci Technol 1988: 22: 263–271.
-
Sekizawa Due south.I., and Tsubone H. Nasal receptors responding to noxious chemical irritants. Respir Physiol 1994: 96 (one): 37–48.
-
Silverman L., Whittenberger J.Fifty., and Muller J. Physiological response of man to ammonia in depression concentrations. J Ind Hyg Toxicol 1949: 31: 74–78.
-
Tanner RL An ambient experimental written report of stage equilibrium in the atmospheric system: aerosol H+, NH+four, SO2−4, NO−3-NH3(1000), HNO3(one thousand) . Atmos Environ 1982: 16 (12): 2935–2942.
-
Verberk G.Thou. Effects of ammonia in volunteers. Int Arch Occup Environ Health 1977: 39: 73–81.
-
Vigliani E.C., and Zurlo N. Experiences of the clinical del Lavero with maximum allowable concentrations of industrial poisons. Arch Gewerbepath U Gewerbehyg 1956: 13: 528–535.
-
Watson Due west.A., Litovitz T.Fifty., Rodgers G.C., Klein-Schwartz West., Youniss J., Rose S.R., Borys D., and May 1000.E. 2002 Annual report of the American Association of Poisonous substance Command Centers Toxic Exposure Surveillance System. Am J Emerg Med 2003: 21 (5): 353–421.
-
Webb A.G., Singer B.C., and Nazaroff Westward.W. Issue of gaseous ammonia on nicotine sorption. Proceedings, Indoor Air 2002 Conference, 2002, pp. 512–517.
-
WHO (Earth Health Organization). Ammonia Environmental Wellness Criteria, Vol. 54. World Health Organisation, Geneva, Switzerland, 1986.
Acknowledgements
Special cheers to Roxanne Agredano and Richard Richter for their valuable assistance. The measurement studies were funded by the legal counsel for an industrial defendant to characterize mutual consumer exposures to ammonia. Neither of the products tested was at issue in the litigation and no funding was received from cleaner product manufacturers. The individual authors funded the literature review and cosmos of this manuscript.
Author information
Affiliations
Corresponding writer
Rights and permissions
About this commodity
Cite this article
Fedoruk, Thousand., Bronstein, R. & Kerger, B. Ammonia exposure and run a risk assessment for selected household cleaning product uses. J Expo Sci Environ Epidemiol 15, 534–544 (2005). https://doi.org/10.1038/sj.jea.7500431
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/sj.jea.7500431
Keywords
- household
- cleaners
- ammonia
- exposure
- airborne
- inhalation
Farther reading
Source: https://www.nature.com/articles/7500431
Posted by: bryantbouring.blogspot.com


0 Response to "Where Can I Buy Ammonia For Cleaning"
Post a Comment